As an advocate in PBPK modeling for risk assessment, I want to share with you the latest guidance from the Organization of Economic Cooperation and Development (OECD). I write a lot of reports on PBPK models for clients, and it makes a huge difference to always use the same format to report the goal of the project, the development of the PBPK model and how it will be used. It makes my life easier and obviously the client appreciates being able to follow and understand what was done. These templates and guidelines developed by the OECD and other regulatory agencies should be use in every project involving PBPK modeling, even if it is not for a regulatory submission. Let’s look at the latest guidance by the OECD which focuses on parameterizing and validating PBPK models when in vivo kinetic data are limited or not available. I have summarized the most interesting take-away’s below.
PBPK modeling is an efficient and reliable tool to predict the fate of chemicals within an organism. PBPK models include biologically realistic descriptions of tissues and processes involved in the absorption, distribution, metabolism, and excretion (ADME) of a compound.
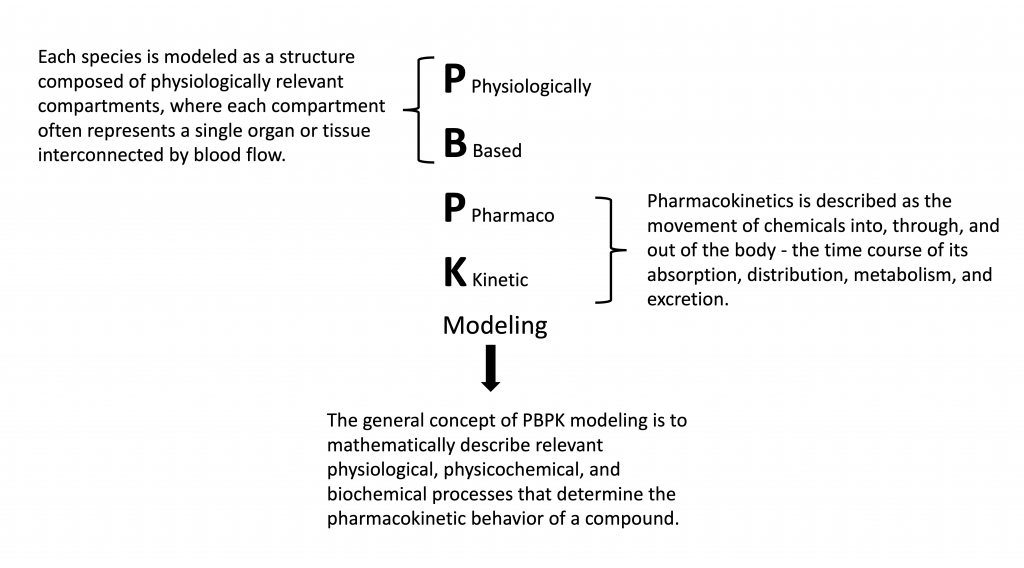
Figure 1. What is PBPK Modeling?
Used in combination with state-of-the-art quantitative structure-activity relationship (QSAR), read-across, and in vitro testing technologies, PBPK modeling serves as a tool to predict toxicity and assess safety of chemicals.
PBPK modeling is well suited for combining the data from these various sources and making predictions of resulting internal dosimetry that can be compared to biomonitoring data to derive MOE.
The guidance from the organization for economic co-operation and development (OECD) is as follows:
- PBPK models are complex and before being used confidently in decision-making context, it is important that the model structure, parameters used, and performance of the model are evaluated.
- Model documentation is essential for evaluation. The Guidance document on the characterization, validation and reporting of Physiologically Based Kinetic (PBK) models for regulatory purposes is a harmonized reporting template to facilitate more efficient and consistent submission and review of model analysis.
- This new guidance is built on existing guidance on model application in risk assessment or good modeling practices from the US-environmental agency (US-EPA), the world health organization (WHO) or the European food safety authority (EFSA).
- As risk assessment is shifting from animal testing to new approaches based on in vitro and in silico methods, alternative strategies are needed to accurately integrate and use these non-animal data. This guidance document is focusing on parameterizing and validating PBPK models when in vivo kinetic data are limited or not available, especially for use in risk assessment.
Below are the key features of this new PBPK modeling guidance:
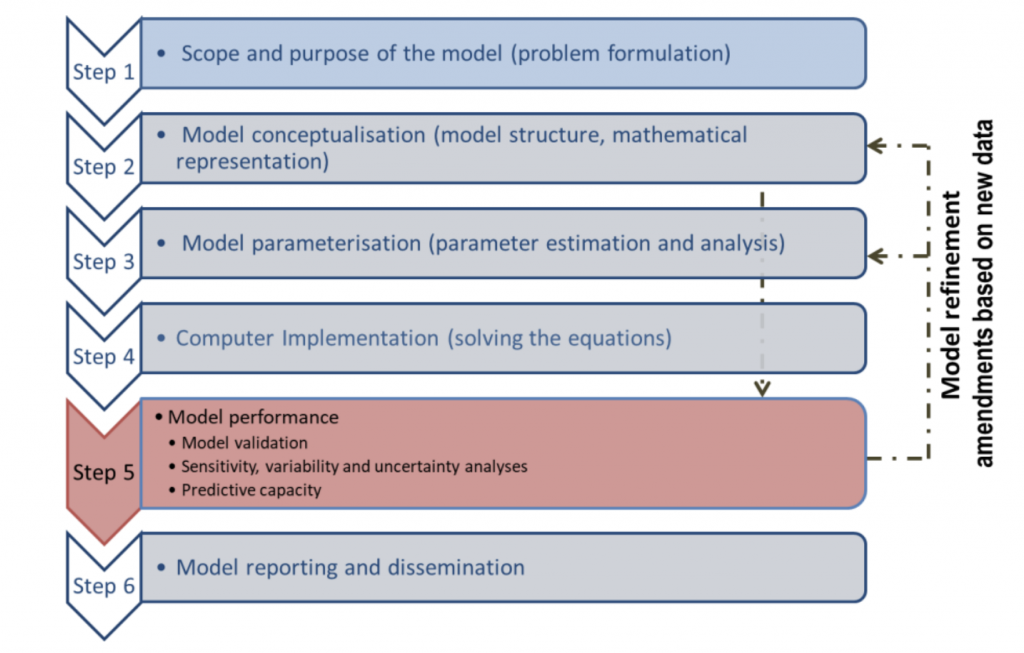
Figure 2. Workflow for model development, validation, reporting and dissemination (OECD Guidance)
- The guidance follows a specific workflow to ensure the validity and applicability of the PBPK model, according to the context of use. The specific question that the model addresses (Step 1) is essential as it draws the model conceptualization and evaluation.
- In the context of the reduction of animal experimentation, or even its ban in Europe for some compound classes, the toolbox for filling toxicity data gaps is limited. The new guidance explains how to establish model validity in these case scenarios by using read-across approaches. This guidance is highlighting the use of in vitro and in silico data to parameterize a PBPK model and how to evaluate such models. The document provides an overview of in silico and in vitro methods for generating parameters used in a PBPK model. They also provide some references for guidelines on generating in vitro data that are reproducible and reliable. The way in silico and in vitro data are generated is essential to ensure the quality of model input and therefore the trust in the PBPK model simulations.
- The guidance is also providing a template for reporting the characteristics of PBPK models as well as a checklist to ensure the completeness of documentation on PBPK models. Some case studies are also provided, illustrating the practical application of the guidance.
If you wish to discuss PBPK modeling and how we can apply it to solve your business problems, email me at mmoreau@scitovation.com.
